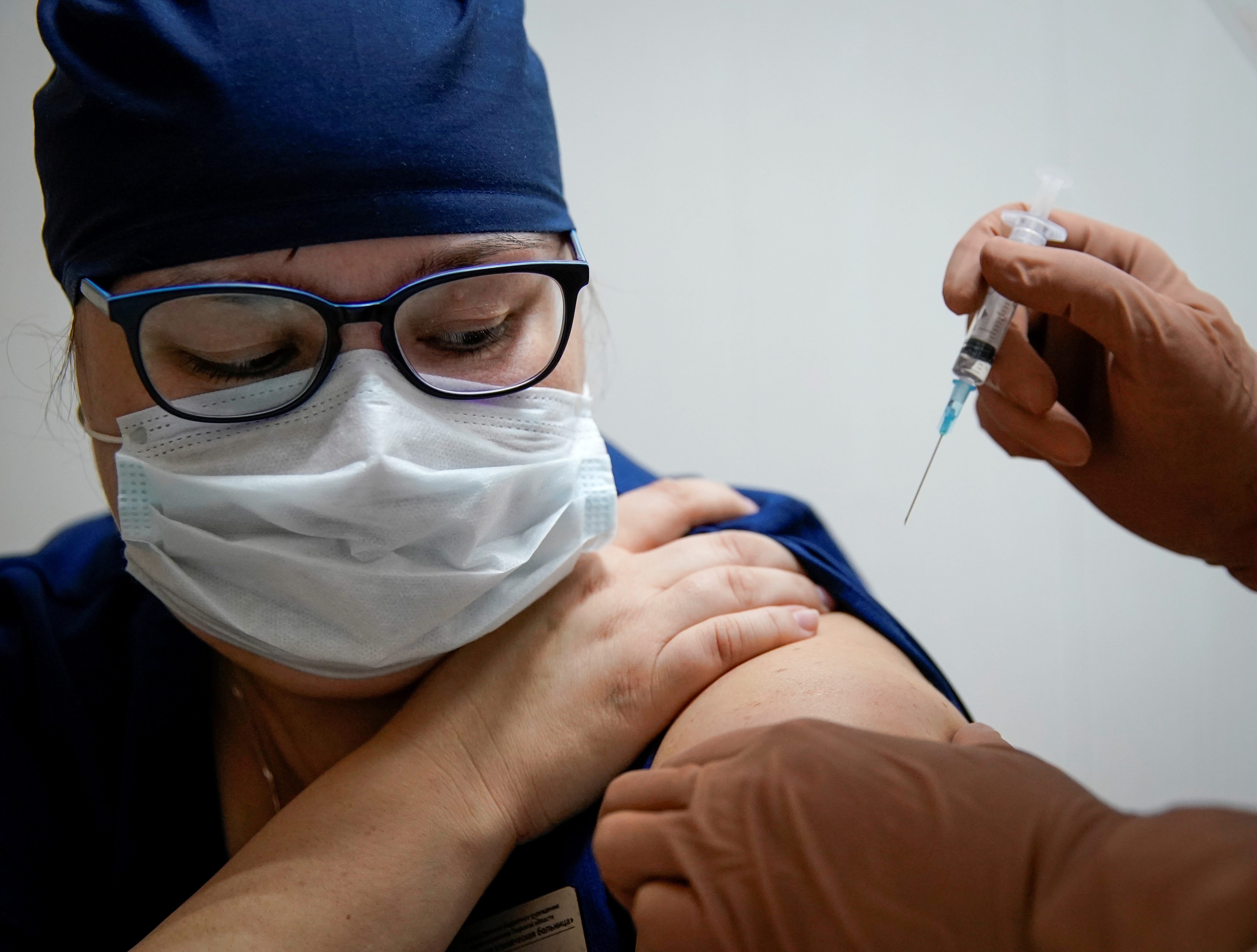
(ATF) Asian pharmaceutical firms are joining the fray to find treatments and vaccines for coronavirus.
Days after Pfizer fired up markets and hopes of an end to the pandemic with the announcement that its candidate vaccine was 90% effective, Japanese and Chinese firms have announced their own investments into remedies.
Fujitsu, Mizuho Financial Group, and drugmaker PeptiDream of Japan said on Thursday they are forming a joint venture to develop treatments for Covid-19.
The Asia Eight: Daily must-reads from world’s most dynamic region
The new company, to be called PeptiAID, will research and develop peptide therapeutics for the current SARS-CoV-2 virus and potential future coronavirus outbreaks, the companies said in a release.
The joint venture also includes Takenaka Corp and Kishida Chemical Co.
Meanwhile, China’s Tibet Rhodiola Pharmaceutical Holding announced a deal on Wednesday to manufacture, sell and test Russia’s vaccine in China, hours after interim results showed it was 92% effective at protecting people from the disease.
The initial results of the Sputnik V vaccine are only the second to be published from a late-stage human trial in the global effort to produce vaccine that could halt a pandemic that has killed more than 1.2 million people and ravaged the world economy.
Rhodiola said it plans to conduct early and mid-stage trials of the Russian vaccine in China and final-stage trials overseas, although the trials are yet to be approved by regulators.
Read More Vaccines News:
- China vaccine stocks take a beating amid foreign doubts
- Sinovac trial suspension complicates vaccine race for China
- Vaccine success seen as breakthrough in fight against virus
Rhodiola, currently unable to produce the Russian vaccine, said it would outsource early development and manufacturing work, and would also consider building production lines at its subsidiary.
The deal requires Rhodiola’s unit to supply the Russian firm with enough vaccine doses to inoculate at least 20 million people in 2021. It adds to several manufacturing deals Russia has announced so far, including a plan to produce 300 million doses in India.
The agreement would also allow Rhodiola to supply vaccine produced in China to designated buyers overseas.
While China has five vaccines in the final stage of clinical trials, local firms have also partnered with foreign drugmakers to test and distribute front-running vaccines in the country.
Shanghai Fosun Pharmaceutical is seeking regulatory approval to trial a product from Pfizer and BioNTech, and Shenzhen Kangtai Biological Products aims to begin human tests for AstraZeneca’s candidate this year.
- With additional reporting by Reuters