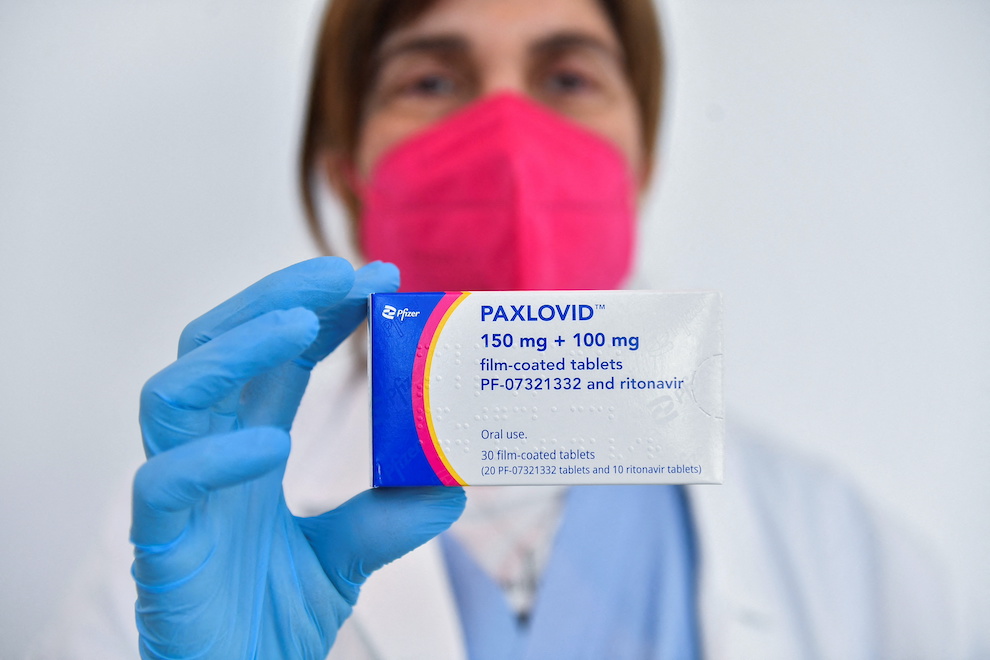
China’s medical products regulator said on Saturday it has given conditional approval for Pfizer’s Covid-19 treatment Paxlovid, making it the first oral pill specifically developed to treat the disease cleared in the country.
The National Medical Products Administration said Paxlovid is approved to treat adults who have mild to moderate Covid-19 and high risk of progressing to a severe condition. Further study on the drug needed to be conducted and submitted to the authority, it said.
It is not immediately clear if China is already in talks with Pfizer to procure the pill.
“This is an important milestone in our fight against Covid-19,” a Pfizer representative said in a statement, without providing information about procurement.
The approval is a boost to Pfizer which expects $22 billion in 2022 sales of the treatment.
Pfizer executives said the company is in active discussions with over 100 countries about Paxlovid, and has the capacity to provide 120 million courses if needed.
While a number of vaccines are available worldwide to help prevent infection and serious illness, including one made by Pfizer, there are limited treatment options for people infected with Covid-19.
Pfizer said in December that final trial results showed its treatment reduced the chance of hospitalisation or death by 89% in Covid patients at risk of severe illness given the treatment within three days of the onset of symptoms, and by 88% when given within five days of onset.
The United States is paying around $530 for each course of Paxlovid and $700 for each course of rival Covid pill molnupiravir developed by Merck & Co.
China has kept daily number of new Covid-19 patients with confirmed symptoms to below 250, and sometimes fewer than 10, in the past year.
The number is small for its 1.4 billion population and by global standards, thanks to China’s approach of quickly containing any local flare-ups as soon as possible and its weeks-long quarantine requirement for most travellers arriving from abroad.
China has yet to approve any Covid-19 vaccines developed by foreign drugmakers but has vaccinated 87.1% of its entire population by Feb. 7 using several domestically developed shots.
Block on Non-Essential Travel
China’s National Immigration Administration said it will not be renewing passports for non-essential travel while the international Covid epidemic is still severe and cross border travel poses “great security risks”.
The administration said in a statement on social media it will “normally issue passports” for individuals who need to travel abroad for study, employment, or business.
But rumours it will issue passport renewals for individuals looking to travel abroad for leisure are false, the authority said.
China has tightened controls over its citizens’ cross-border travel to lower the risk of the virus being brought in from overseas and spreading domestically.
It has suspended the issuance of new common passports for individuals who intend to go abroad for non-urgent matters.
• Reuters with additional editing by Jim Pollard
This report was updated with further details on February 12, 2022.
ALSO on AF:
China Vows to Support Hong Kong as Covid-19 Cases Hit Record
Shionogi & Co Shares Rise on Covid-19 Pill Trial Results
China’s Zero Covid Policy to Unsettle Markets This Year: Eurasia
China Study Warns Of Huge Covid Outbreak If It Opens Up